Implementing the Gene Technology Scheme: The Federal Department of Health’s art of making the possible impossible.
12 September 2022
In due course…
Australia has seen some major public policy achievements in the last few years, but an up-to-date regulatory framework for agricultural biotechnology is definitely not one of them.
At the appropriate juncture…
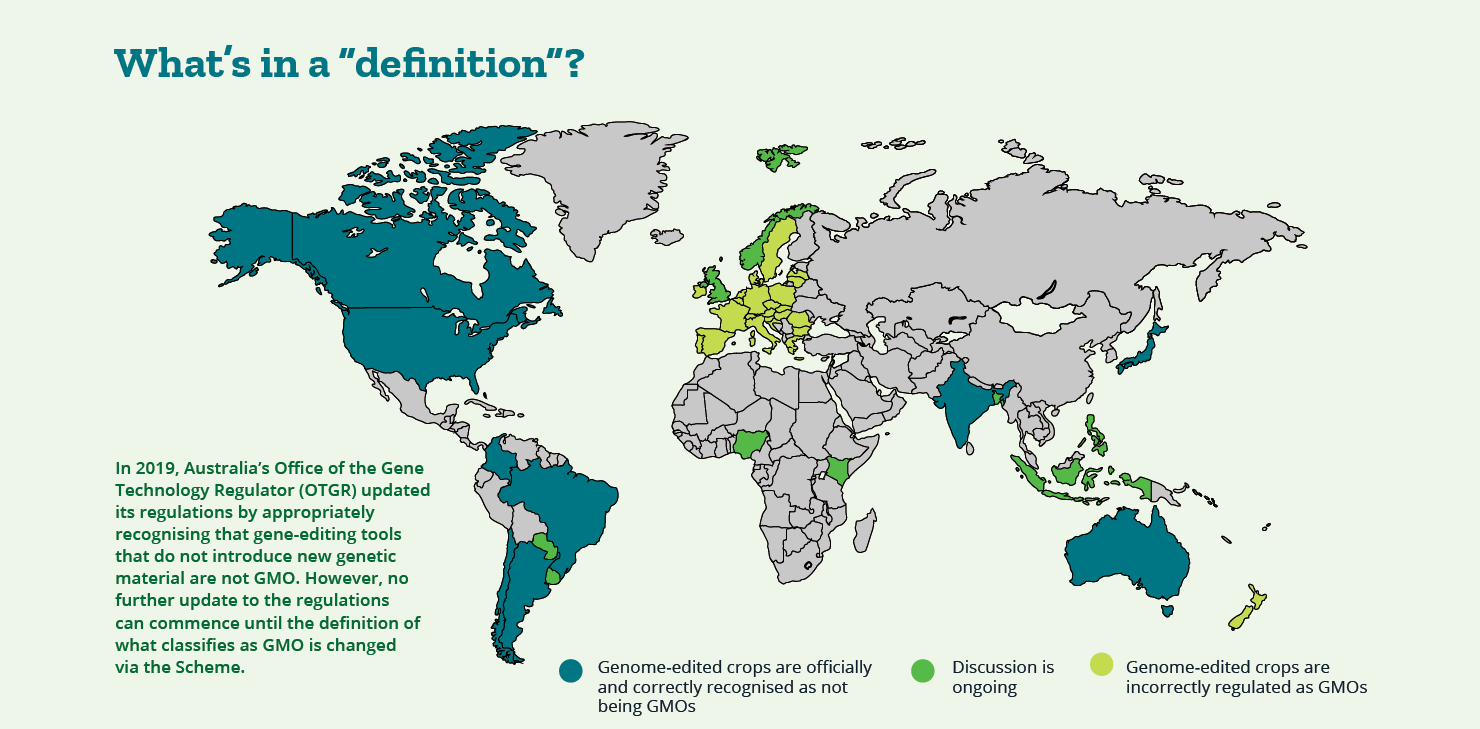
The 2017 Review of the National Gene Technology Scheme started as a routine assessment to ensure the current regulation of biotechnology meets its purpose and keeps up with advances in gene technology. After three rounds of public consultation, an Action Plan was produced in 2018, requiring (yes you guessed it) a further two rounds of stakeholder consultation before implementation. Since then, no meaningful progress has been actioned, lapsing well beyond implementation deadlines. It begs the question, have we been catfished by the Federal Department of Health bureaucracy?
When all the necessary procedures have been completed…
Fit-for-purpose regulatory reform listens to scientific consensus as it evolves over time and the stakeholders who use it. Mere consultation for consultation’s sake has made for good satire over the years but not good policy. Even Einstein said, “Bureaucracy is the death of all sound work”. At this rate, current recommendations won’t be implemented before another Review is due to start this year.
When the moment is ripe…
If implemented, the Third Review of the National Gene Technology Scheme presents an opportunity to remain a world-leader in biotechnology regulation and build sovereign capacity. This will not be achieved by an idle system that creates the very legislative stalemate it was established to avoid. Rather, a clear future-proofed system that keeps agricultural and medical innovations onshore will prepare Australia for the almost certain challenges we face but don’t yet see.